Taiwan AI Labs Partners With Shin-Kong Hospital for Ensuring Quality in Clinical Application of NGS
Medical care in cancer treatment planning and inherited disease diagnosis have increasingly adopted genetic-based examination as part of standard medical care. At the beginning of 2021, the Taiwan Food and Drug Administration(TFDA) modified the “Regulations Governing the Application or Use of Specific Medical Techniques or Examinations, or Medical Devices,” which added the guidance of the regulatory approach to genetic testing. Guidelines specifically describe how clinical laboratories design, develop, and validate tests. Also, it identifies the role and responsibility of healthcare institutes for adopting these new testing in the patient journey.
Among the varieties of genetic testing technology, NGS has the potential to bring vast amounts of data, meaning that efficient systems for storage and QC evaluation are more essential than ever. TAIGenomics, under Taiwan AI Labs, has continued to collaborate with the Precision Medicine Center of Shin Kong Hospital(SKH) over the past three years to implement a clinical-user-friendly platform called QCheck into their medical flow. This platform follows up different guidelines and regulations, such as FDA, ACMG, NYSDOH, and CAP, and provides different perspectives to monitor the quality of NGS-based genetic testing.
“TAIGenomics is delicate in combining the power of informatics talents and clinicians. In the future of precision medicine, we want to provide the security of recognized certification and ensure clinical labs deliver optimum performance, quality, and safety in sensitive clinic-genomic data,” Taiwan AI Labs Founder Ethan Tu said in a statement.
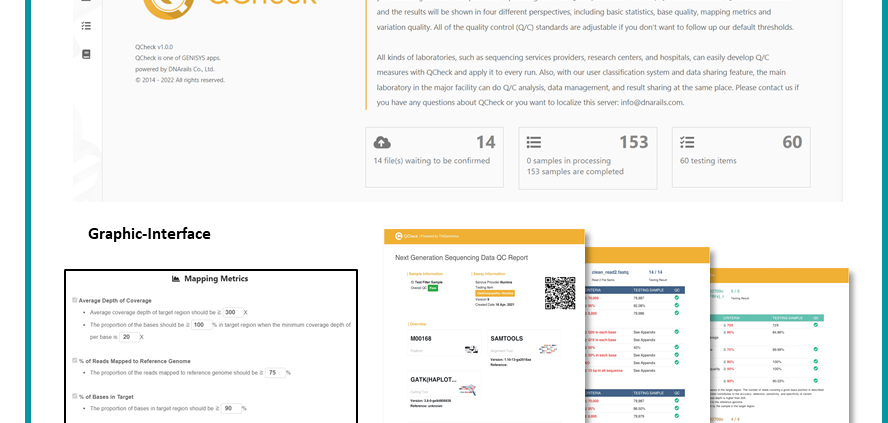
↑ QCheck (Accumulate and Validate your omics data quality with trusted international guidelines): Developed by Taiwan AI Labs with graphic-interface, comprehensive quality item, and automatic reports for clinicians and pathologists. Assist with the omics data quality check and comply with LDTs regulations in Taiwan and international guidelines.
“We had set up the precision medicine center in 2018, which aims to accelerate the processing of adopting new technology into the clinical field,” Superintendent from SKH, Sheng-Mou Hou, said in a statement. “With the enhancement of the regulation, healthcare providers and examination laboratories must ensure high levels of data quality and rigorous validation in variant interpretation for the adoption of companion diagnostics.”
“As a gatekeeper in Shin-kong hospital, our mission is to assure testing quality from the clinical laboratories,” Director from Precision Medicine Center ar SKH, Hong-Jhih Lai, said. “We deployed troops in preparation for the regulation. We have already collected and confirmed more than 1,000 cases in the last three years and will keep optimizing the workflow with the TAIGenomics team.”
↑ Smart medical information system: Combined with the information management system and QCheck software, quality control is carried out for the genetic testing items of testing manufacturers. If it doesn’t meet the regulations, the center will request the testing manufacturers to re-test.
Errors will occur in all areas of laboratory testing. When it happens in genetic testing, it can prompt clinical decisions with a high risk since it’s not easy to be identified. As genetic testing has become the future of medicine, all stakeholders in precision medicine industries should think beyond the baseline standard and start working on a more high-quality validation system.
台灣人工智慧實驗室攜手新光醫院 實現病患個人化醫療
癌症治療及遺傳疾病已經跨入精準醫療時代,為了提升台灣疾病檢測及臨床服務的品質,衛福部於110年2月9日發布了修正版特管辦法,因應醫療服務品質及醫療技術的發展,將實驗室開發檢測(Laboratory Developed Tests, LDTs)也納入其中。台灣人工智慧實驗室(Taiwan AI Labs)與新光醫院精準醫學中心透過臨床及技術的雙向合作,超前部署開發出精準醫學中心之「智慧醫療資訊系統」,針對多項實驗室開發檢測,特別是次世代定序檢測,進行自動化品管驗證,新光醫院成為台灣首家醫學中心將基因檢測品質導入第三方數據品質管理的概念,為病患的檢測服務優先把關。
台灣人工智慧實驗室創辦人杜奕瑾表示,以台灣強大的軟實力加上醫學中心寶貴的臨床經驗,結合資訊管理的創新能力及臨床實際的需求,我們走在特管法之前,即透過國際標準為原則,針對各家廠商開發出的檢測做第三方的把關,作為醫療品質和數據品質的守門員,讓患者能得到高品質且適當的服務,則是此系統的初衷,在累積這些高品質把關下的結果,未來實驗室將會繼續與新光醫院合作,透過AI技術來建立精準醫療的解決方案。
新光醫院院長侯勝茂坦言隨著醫療科技進步及環境變遷影響,許多公司加入診斷療法的研發,精準醫療與大數據的應用也愈來愈多元;新光醫院於2018年成立了精準醫學中心,就是希望能夠促進個人化醫療服務,而法規的修正則更增加了實驗室開發檢測的嚴謹度,讓品質優良的數據能提供更精準的判讀結果。具多年臨床經驗的精準醫學中心暨血液腫瘤科主任賴泓誌醫師指出,醫院在為病患選擇最佳療法,需以病患權益出發作考量,還要同時做好品質及臨床的控管,才能避免對檢測結果的錯誤解讀而影響病人的治療。過去這兩年以來,新光醫院使用此系統共檢查了一千多筆檢測報告的數據品質,同步儲存原始檢測資料,提前為LDTs法規檢核標準做準備。隨著醫院擴展檢測項目,以及檢體送檢的數量增加的情況下,此定序品質資訊系統確實提供了第三方的品管認證作為檢測數據品質好壞的判斷依據。
近年來個人化醫療意識逐漸增加,根據 Research and Markets 統計,2025年以前全球精準醫療市場規模將達到 1,126 億美金。因此現今的基因檢測已不限於癌症治療,針對新生兒、遺傳疾病、代謝疾病、個人健康與老化等,皆有多元的產品及服務推出,該如何選擇最佳的診斷及為個人基因數據的把關,則應透過智慧化的檢測品質資訊系統,協助解決臨床實際面臨的問題。未來精準醫療服務,要如何減輕病人身心負擔、節省醫療資源及持續創新產品,健全的法規、醫療、科技三者的結合是必然的趨勢。